EE 372 Assignment 2 Solutions
EE 372 Assignment 2 Solutions
Download the Jupyter notebook here.
%matplotlib inline
# The only libraries you should need for this assignment.
# Execute this cell first. (You should get a sine wave.)
import matplotlib.pyplot as plt
import numpy as np
x = np.arange(0,1.0, 0.01)
y = np.sin(2*np.pi*x)
plt.plot(x,y)
plt.grid()
plt.xlabel('x')
plt.ylabel('y')
plt.title('Example plot')
plt.show()
Question I: Validity of Lander-Waterman Statistics
1.
You can view the first 15 lines of the file using the command samtools view ecoli_aligned_reads.bam | head -15. Refer to the documentation for the *.sam file format here. Convert the *.bam file to a *.sam file using the samtools view command. What position does the first read align to?
The first read aligns to position 1850828.
2.
The length of the E. coli genome is 4639675. Write a function to compute the proportion of the genome by covered by the reads in a given *.sam file. What proportion of the genome is covered by the reads in ecoli_aligned_reads.bam?
def compute_proportion_covered(filename):
covered = np.zeros(4639675)
read_length = 150
with open(filename) as f:
for line in f:
if line[0] != '@':
pos = int(line.split()[3])-1
covered[pos:pos+read_length] = 1
return np.sum(covered)/float(len(covered))
p_covered = compute_proportion_covered('ecoli_aligned_reads.sam')
print('%.2f%% of the genome is covered.'%(100*p_covered))
99.47% of the genome is covered.
3.
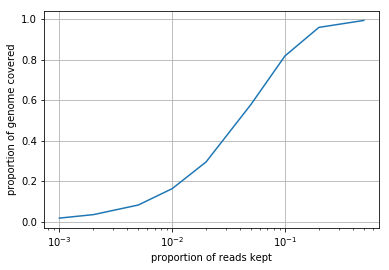
Subsample the *.sam file using the samtools view -s command. You should generate a new *.sam file for each subsampling. Using the function you wrote above, plot the proportion of the genome covered as a function of \(p\), the proportion of reads kept. Interpret what you see (1-2 sentences).
We see that as the proportion of reads kept decreases, so does the proportion of the genome covered. This relationship is not linear, however, as throwing away half the reads results in only a minor decrease in coverage. The rate of genome coverage gain per read decreases as the number of reads increases.
import os
p_reads = [0.001, 0.002, 0.005, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5]
p_covered = np.zeros(len(p_reads))
for i, p in enumerate(p_reads):
samp_filename = 'ecoli_aligned_reads_p%s.sam'%(p)
# subsample the sam file
cmd = 'samtools view -s %s ecoli_aligned_reads.sam > %s'%(p, samp_filename)
os.system(cmd)
p_covered[i] = compute_proportion_covered(samp_filename)
plt.plot(p_reads, p_covered)b
plt.xscale('log')
plt.xlabel('proportion of reads kept')
plt.ylabel('proportion of genome covered')
plt.grid()
4.
Compute the triple repeat and interleaved statistics of the E. coli genome (available here). Report the number of triple and interleaved repeats of length more than 200. Consider only the forward strand of the reference. Hint: Use the software MUMmer. The repeat-match command might be helpful.
We first run the command repeat-match -f -n 200 E.coli_K12_ATCC_700926.fasta > ecoli_repeats.txt to get the positions of all repeats in the genome. Each line in the repeat file contains a repeat (e.g. the start position of the 1st copy of the repeat, and the start position of the 2nd copy of the repeat, and the length of each copy).
We then post process the repeat file. The post processing compares all possible pairs of repeats. It tries to find three patterns:
- interleaved repeat: r1=…x…x…, r2=…y…y…, and r1 plus r2 has …x…y…x…y…
- triple repeat: r1=…x…x…, r2=…x…x…, and r1 plus r2 has …x…x…x…
- interleaved-triple hybrid: r1=…zx…zx…, r2=…yz…yz…, r1 plus r2 has …zx…yzx…yz…
def find_int_trip_repeat(repeat_list):
trip_repeats = {}
trip_repeat_S = []
l_trip = -1
int_repeats = {}
int_repeat_S = []
l_int = -1
int_cnt = 0
trip_cnt = 0
overlap_cnt = 0
for i1 in range(len(repeat_list)):
for i2 in np.arange(i1+1, len(repeat_list)):
repeat1 = repeat_list[i1]
p1_1, p1_2, L1 = repeat1
p1_1e = p1_1+L1-1
p1_2e = p1_2+L1-1
repeat2 = repeat_list[i2]
p2_1, p2_2, L2 = repeat2
p2_1e = p2_1+L2-1
# pattern 1: ...x...x.....
# .....y...y...
if p1_1e<p2_1 and p2_1e<p1_2 and p1_2e<p2_2:
l_int_new = min(L1,L2)
r = int_repeats.setdefault(l_int_new, [])
r.append([repeat1, repeat2])
int_cnt += 1
if l_int_new>l_int:
l_int = l_int_new
int_repeat_S = [repeat1, repeat2]
# pattern 2: ...x...x.......
# .......x...x...
if L1==L2 and p1_2==p2_1:
r = trip_repeats.setdefault(L1,[])
r.append([repeat1, repeat2])
trip_cnt += 1
if L1>l_trip:
l_trip = L1
trip_repeat_S = [repeat1, repeat2]
# pattern 3: ...zxAyzxByz...
# ...zx..zx......
# ......yz..yz...
if p1_1e<p2_1 and p2_1<p1_2 and p2_1e>p1_2 and p2_1e<p1_2e and p1_2e<p2_2:
overlap_cnt += 1
L_z = p2_1e-p1_2+1
L_x = L1-L_z
L_y = L2-L_z
#...z...z...z
repeat_z_1 = [p1_1, p1_2, L_z]
repeat_z_2 = [p1_2, p2_2+L_y, L_z]
r = trip_repeats.setdefault(L_z,[])
r.append([repeat_z_1, repeat_z_2])
trip_cnt += 1
if L_z>l_trip:
l_trip = L_z
trip_repeat_S = [repeat_z_1, repeat_z_2]
#...x.y.x.y...
repeat_xy_1 = [p1_1+L_z, p1_2+L_z, L_x]
repeat_xy_2 = [p2_1, p2_2, L_y]
L_xy = min(L_x, L_y)
r = int_repeats.setdefault(L_xy, [])
r.append([repeat_xy_1, repeat_xy_2])
int_cnt += 1
if L_xy>l_int:
l_int = L_xy
int_repeat_S = [repeat_xy_1, repeat_xy_2]
print('Max-length interleaved repeats:')
print(' repeats at pos %s and %s (length %s)\n'%int_repeat_S[0] + \
' repeats at pos %s and %s (length %s)'%int_repeat_S[1])
print('Max-length triple repeat:')
print(' repeats at pos %s, %s, and %s (length %s)' \
%(trip_repeat_S[0][0], trip_repeat_S[0][1], trip_repeat_S[1][1], l_trip))
print('Total interleaved repeats = %s (%s from hybrids)'%(int_cnt, overlap_cnt))
print('Total triple repeats = %s (%s from hybrids)'%(trip_cnt, overlap_cnt))
# extract list of repeats
repeat_list = []
with open('ecoli_repeats.txt') as rf:
for i, line in enumerate(rf):
if i > 1:
r_copy1_pos, r_copy2_pos, r_len = [int(j) for j in line.split()]
# only keep repeats that don't overlap
if r_copy1_pos+r_len-1 < r_copy2_pos:
repeat_list.append((min(r_copy1_pos, r_copy2_pos),
max(r_copy1_pos, r_copy2_pos), r_len))
find_int_trip_repeat(sorted(repeat_list))
Max-length interleaved repeats:
repeats at pos 4164672 and 4206160 (length 1566)
repeats at pos 4166642 and 4208044 (length 2815)
Max-length triple repeat:
repeats at pos 15387, 607230, and 2512295 (length 1345)
Total interleaved repeats = 2748 (10 from hybrids)
Total triple repeats = 73 (10 from hybrids)
Question II: de Bruijn graph assembly
1.
Give the 5-mer spectrum of TAAAAACCCCAAAAAG. How many different assemblies are consistent with the 5-mer spectrum?
Because of the ambiguity associated with the two AAAAA k-mers, there are three possible assemblies:
- TAAAAACCCCAAAAAG
- TAAAAAACCCCAAAAG
- TAAAACCCCAAAAAAG
from collections import Counter
st = 'TAAAAACCCCAAAAAG'
list_of_reads = [st[i:i+5] for i in range(len(st)-4)]
kspec = Counter(list_of_reads)
for kmer in kspec:
print('%s appears %s time(s).'%(kmer, kspec[kmer]))
AACCC appears 1 time(s).
AAACC appears 1 time(s).
CCCAA appears 1 time(s).
TAAAA appears 1 time(s).
CCAAA appears 1 time(s).
ACCCC appears 1 time(s).
CCCCA appears 1 time(s).
AAAAA appears 2 time(s).
AAAAC appears 1 time(s).
CAAAA appears 1 time(s).
AAAAG appears 1 time(s).
2.
The support of a k-mer spectrum is the k-mer spectrum with the value of all non-zero k-mers set to 1. Give the assembly of TAAAAACCCCAAAAAG from the support of its 5-mer spectrum. How many different assemblies are consistent with the support of this 5-mer spectrum?
We now have 1 AAAAA k-mer instead of two, and therefore there are 2 different assemblies consistent with the support of this 5-mer spectrum:
- TAAAACCCCAAAAAG
- TAAAAACCCCAAAAG
3.
Study the implementation of the de Bruijn graph assembler by Ben Langmead here. You should copy and paste the code from the top cell into your notebook as you will use this to class to perform assembly. Note that you will need to pass a list of reads (strings) as the strIter argument when initializing an instance of the class (see __iter___). You can use the eulerianWalkOrCycle method to obtain a list of \(k-1\)-mers corresponding to an Eulerian walk through the graph. Write a function that obtains the assembly from this list of \(k-1\)-mers (i.e. if the list is [‘ABCD’,’BCDE’] with \(k=5\), then your function should return ‘ABCDE’).
class DeBruijnGraph:
''' De Bruijn directed multigraph built from a collection of
strings. User supplies strings and k-mer length k. Nodes
are k-1-mers. An Edge corresponds to the k-mer that joins
a left k-1-mer to a right k-1-mer. '''
@staticmethod
def chop(st, k):
''' Chop string into k-mers of given length '''
for i in range(len(st)-(k-1)):
yield (st[i:i+k], st[i:i+k-1], st[i+1:i+k])
class Node:
''' Node representing a k-1 mer. Keep track of # of
incoming/outgoing edges so it's easy to check for
balanced, semi-balanced. '''
def __init__(self, km1mer):
self.km1mer = km1mer
self.nin = 0
self.nout = 0
def isSemiBalanced(self):
return abs(self.nin - self.nout) == 1
def isBalanced(self):
return self.nin == self.nout
def __hash__(self):
return hash(self.km1mer)
def __str__(self):
return self.km1mer
def __init__(self, strIter, k, circularize=False):
''' Build de Bruijn multigraph given string iterator and k-mer
length k '''
self.G = {} # multimap from nodes to neighbors
self.nodes = {} # maps k-1-mers to Node objects
for st in strIter:
if circularize:
st += st[:k-1]
for kmer, km1L, km1R in self.chop(st, k):
nodeL, nodeR = None, None
if km1L in self.nodes:
nodeL = self.nodes[km1L]
else:
nodeL = self.nodes[km1L] = self.Node(km1L)
if km1R in self.nodes:
nodeR = self.nodes[km1R]
else:
nodeR = self.nodes[km1R] = self.Node(km1R)
nodeL.nout += 1
nodeR.nin += 1
self.G.setdefault(nodeL, []).append(nodeR)
# Iterate over nodes; tally # balanced, semi-balanced, neither
self.nsemi, self.nbal, self.nneither = 0, 0, 0
# Keep track of head and tail nodes in the case of a graph with
# Eularian walk (not cycle)
self.head, self.tail = None, None
for node in iter(self.nodes.values()):
if node.isBalanced():
self.nbal += 1
elif node.isSemiBalanced():
if node.nin == node.nout + 1:
self.tail = node
if node.nin == node.nout - 1:
self.head = node
self.nsemi += 1
else:
self.nneither += 1
def nnodes(self):
''' Return # nodes '''
return len(self.nodes)
def nedges(self):
''' Return # edges '''
return len(self.G)
def hasEulerianWalk(self):
''' Return true iff graph has Eulerian walk. '''
return self.nneither == 0 and self.nsemi == 2
def hasEulerianCycle(self):
''' Return true iff graph has Eulerian cycle. '''
return self.nneither == 0 and self.nsemi == 0
def isEulerian(self):
''' Return true iff graph has Eulerian walk or cycle '''
# technically, if it has an Eulerian walk
return self.hasEulerianWalk() or self.hasEulerianCycle()
def eulerianWalkOrCycle(self):
''' Find and return sequence of nodes (represented by
their k-1-mer labels) corresponding to Eulerian walk
or cycle '''
assert self.isEulerian()
g = self.G
if self.hasEulerianWalk():
g = g.copy()
g.setdefault(self.tail, []).append(self.head)
# graph g has an Eulerian cycle
tour = []
src = next(iter(g.keys())) # pick arbitrary starting node
def __visit(n):
while len(g[n]) > 0:
dst = g[n].pop()
__visit(dst)
tour.append(n)
__visit(src)
tour = tour[::-1][:-1] # reverse and then take all but last node
if self.hasEulerianWalk():
# Adjust node list so that it starts at head and ends at tail
sti = tour.index(self.head)
tour = tour[sti:] + tour[:sti]
# Return node list
return list(map(str, tour))
def get_assembly(list_of_nodes):
return ''.join([list_of_nodes[0]] + \
[list_of_nodes[i][-1]
for i in range(1, len(list_of_nodes))])
k = 5
g = DeBruijnGraph(list_of_reads, k)
list_of_nodes = g.eulerianWalkOrCycle()
get_assembly(list_of_nodes)
'TAAAAACCCCAAAAAG'
4.
Write a function to generate random reads. The input should be the number of reads generated \(N\) and the length \(L\) of each read generated. The output should be \(N\) random length-\(L\) sequences of nucleotides. Generate a random length-100 genome.
def generate_reads(N, L):
return [''.join(np.random.choice(['A', 'C', 'G', 'T'], L))
for i in range(N)]
np.random.seed(2)
g = generate_reads(1, 100)[0]
print(g)
ATCAGTGTATGCTTCTTTGAAACTTGAGTTTGGCGATTCAAGGTTCTGAAGAGAGTTCTTGAGCGTCCATCGAGCCCCGAGGTATAGGAAGGAGACGCGG
5.
Write a function to sample reads from a genome. The input should be the genome, the number of reads generated \(N\), and the length \(L\) of each read generated. Assuming that \(L = 10\), how many reads do you need to achieve a coverage depth of 30? Generate this number of reads and give the assembly using your code from part 3.
def sample_reads_from_genome(genome, N, L):
lmers = [genome[i:i+L] for i in range(len(genome)-L+1)]
return np.random.choice(lmers, N)
L = 10
c = 30
N = len(g)*c/L
print('N = %s reads are needed to achieve a coverage depth of %s.'%(N, c))
reads = sample_reads_from_genome(g, N, L)
unique_reads = Counter(reads)
print('\nReads generated: ')
for ur in sorted(unique_reads):
print(' %s appears %s time(s).'%(ur, unique_reads[ur]))
print('\nOriginal genome: %s'%(g))
dbg = DeBruijnGraph(np.unique(reads), L)
list_of_nodes = dbg.eulerianWalkOrCycle()
g_hat = get_assembly(list_of_nodes)
print('\nAssembled genome: %s'%(g_hat))
N = 300 reads are needed to achieve a coverage depth of 30.
Reads generated:
AAACTTGAGT appears 6 time(s).
AACTTGAGTT appears 2 time(s).
AAGAGAGTTC appears 1 time(s).
AAGGAGACGC appears 1 time(s).
AAGGTTCTGA appears 3 time(s).
ACTTGAGTTT appears 8 time(s).
AGAGAGTTCT appears 8 time(s).
AGAGTTCTTG appears 5 time(s).
AGCCCCGAGG appears 2 time(s).
AGCGTCCATC appears 2 time(s).
AGGAAGGAGA appears 4 time(s).
AGGAGACGCG appears 1 time(s).
AGGTATAGGA appears 7 time(s).
AGGTTCTGAA appears 6 time(s).
AGTGTATGCT appears 1 time(s).
AGTTCTTGAG appears 1 time(s).
AGTTTGGCGA appears 4 time(s).
ATAGGAAGGA appears 6 time(s).
ATCAGTGTAT appears 4 time(s).
ATCGAGCCCC appears 3 time(s).
ATGCTTCTTT appears 5 time(s).
ATTCAAGGTT appears 3 time(s).
CAAGGTTCTG appears 3 time(s).
CAGTGTATGC appears 2 time(s).
CATCGAGCCC appears 1 time(s).
CCATCGAGCC appears 1 time(s).
CCCCGAGGTA appears 3 time(s).
CCCGAGGTAT appears 1 time(s).
CCGAGGTATA appears 3 time(s).
CGAGCCCCGA appears 4 time(s).
CGAGGTATAG appears 1 time(s).
CGATTCAAGG appears 2 time(s).
CGTCCATCGA appears 6 time(s).
CTGAAGAGAG appears 2 time(s).
CTTCTTTGAA appears 4 time(s).
CTTGAGCGTC appears 4 time(s).
CTTGAGTTTG appears 7 time(s).
CTTTGAAACT appears 2 time(s).
GAAACTTGAG appears 2 time(s).
GAAGAGAGTT appears 4 time(s).
GAAGGAGACG appears 3 time(s).
GAGAGTTCTT appears 2 time(s).
GAGCCCCGAG appears 1 time(s).
GAGCGTCCAT appears 1 time(s).
GAGGTATAGG appears 3 time(s).
GAGTTCTTGA appears 2 time(s).
GAGTTTGGCG appears 1 time(s).
GATTCAAGGT appears 5 time(s).
GCCCCGAGGT appears 5 time(s).
GCGATTCAAG appears 1 time(s).
GCGTCCATCG appears 4 time(s).
GCTTCTTTGA appears 4 time(s).
GGAAGGAGAC appears 3 time(s).
GGAGACGCGG appears 6 time(s).
GGCGATTCAA appears 3 time(s).
GGTATAGGAA appears 6 time(s).
GGTTCTGAAG appears 2 time(s).
GTATAGGAAG appears 4 time(s).
GTATGCTTCT appears 5 time(s).
GTCCATCGAG appears 3 time(s).
GTGTATGCTT appears 2 time(s).
GTTCTGAAGA appears 3 time(s).
GTTCTTGAGC appears 3 time(s).
GTTTGGCGAT appears 4 time(s).
TAGGAAGGAG appears 1 time(s).
TATAGGAAGG appears 8 time(s).
TATGCTTCTT appears 2 time(s).
TCAAGGTTCT appears 4 time(s).
TCAGTGTATG appears 4 time(s).
TCCATCGAGC appears 1 time(s).
TCGAGCCCCG appears 3 time(s).
TCTGAAGAGA appears 2 time(s).
TCTTGAGCGT appears 3 time(s).
TCTTTGAAAC appears 2 time(s).
TGAAACTTGA appears 5 time(s).
TGAAGAGAGT appears 2 time(s).
TGAGCGTCCA appears 7 time(s).
TGAGTTTGGC appears 1 time(s).
TGCTTCTTTG appears 1 time(s).
TGGCGATTCA appears 2 time(s).
TGTATGCTTC appears 4 time(s).
TTCAAGGTTC appears 7 time(s).
TTCTGAAGAG appears 6 time(s).
TTCTTGAGCG appears 1 time(s).
TTCTTTGAAA appears 2 time(s).
TTGAAACTTG appears 4 time(s).
TTGAGCGTCC appears 5 time(s).
TTGAGTTTGG appears 4 time(s).
TTGGCGATTC appears 3 time(s).
TTTGAAACTT appears 2 time(s).
TTTGGCGATT appears 3 time(s).
Original genome: ATCAGTGTATGCTTCTTTGAAACTTGAGTTTGGCGATTCAAGGTTCTGAAGAGAGTTCTTGAGCGTCCATCGAGCCCCGAGGTATAGGAAGGAGACGCGG
Assembled genome: ATCAGTGTATGCTTCTTTGAAACTTGAGTTTGGCGATTCAAGGTTCTGAAGAGAGTTCTTGAGCGTCCATCGAGCCCCGAGGTATAGGAAGGAGACGCGG
6.
Write a modified version of the previous function for sampling reads from a genome with error. Generate random length-10 reads with 5% error rate and a coverage of 30. Give the assembly using your code for part 3. What do you observe?
Finding an Eulerian cycle is much harder now. Below, we generate 10000 sets of noisy reads, and none of them were able to be used to find an Eulerian cycle.
def sample_noisy_reads_from_genome(genome, N, L):
G = len(genome)
read_start = np.random.randint(0,G-L,N)
def add_noise(read):
L = len(read)
new_read = ''
noise = np.random.randint(0, 100, L) < 5
for ind, ch in enumerate(read):
ch_pass = ch
if noise [ind]:
if ch == 'A':
ch_pass = np.random.choice(['C','G','T'])
elif ch == 'C':
ch_pass = np.random.choice(['A','G','T'])
elif ch == 'G':
ch_pass = np.random.choice(['A','C','T'])
elif ch == 'T':
ch_pass = np.random.choice(['A','C','G'])
new_read += ch_pass
return new_read
noisy_read_list = [add_noise(genome[index:index+L]) for index in read_start]
return noisy_read_list
ii = 0
while True and ii < 10000:
try:
np.random.seed(ii)
reads = sample_noisy_reads_from_genome(g, N, L)
unique_reads = Counter(reads)
dbg = DeBruijnGraph(np.unique(reads), L)
list_of_nodes = dbg.eulerianWalkOrCycle()
g_hat = get_assembly(list_of_nodes)
print('\nAssembled genome: %s'%(g_hat))
break
except:
ii += 1
Question III: Viterbi Algorithm
The code/solutions for this problem is provided courtesy of Mark Nishimura.
1.
The state space here is \(\{00,01,10,11\}\) . What can you say about the inter-symbol interference? In other words, how many nucleotides are in the pore at each sampling?
There are two nucleotides passing through the pore at each sampling, and therefore the signal from one nucleotide interferes with the signal from the other.
2.
Consider the output sequence \(1.34, 0.23, 1.45, 0.5, 0.11\). Using the above state diagram, compute the sequence obtained by Viterbi decoding. Assume that each observed \(y_i\) is a weighted linear combination of the symbols in the context plus some noise: \(y_i = 0.9 s_i + 0.25 s_{i-1} + n_i\). Assume that the added noise is Gaussian with zero mean.
def ViterbiDecoding2mer(y, Q, S):
"""Performs Viterbi decoding on y, given a matrix
Q of coefficients for [s_i,s_i-1] and an array of states S"""
def prev_state(s, S):
"""Takes a current 2-mer state [s_i, s_i-1] and outputs a list
of possibilities for the previous state:
[s_i-1, 0] and [s_i-1, 1]
"""
curr_index = np.where(np.all(S == s, axis = 1))
s_im1 = S[curr_index, 1].squeeze()
return [np.array([s_im1, 0]), np.array([s_im1, 1])]
def state_to_index(s, S):
return np.where(np.all(S == s, axis=1))[0][0]
def state_to_index(s, S):
return np.where(np.all(S == s, axis=1))[0][0]
trellis = (y - S.dot(Q).reshape((-1,1)))**2 # Matrix of node costs
costs = np.zeros(trellis.shape)
paths = {} # Remember which path went where
# Initialize costs for 0th time step
for i in range(trellis.shape[0]):
costs[i, 0] = trellis[i, 0]
paths[(i, 0)] = None # Signal end of path
for t in range(1, trellis.shape[1]):
# Iterating over time steps
for i in range(trellis.shape[0]):
# Iterating over states:
prev_states = prev_state(S[i,:], S)
# Find the minimum-cost previous-state plus current-state transition,
# and remember which state it is.
min_cost, prev = min((trellis[i, t] + costs[state_to_index(s, S), t-1],
state_to_index(s, S))
for s in prev_states)
paths[(i, t)] = prev
costs[i, t] = min_cost
# Find the min-cost total path by looking at the last column, then using the paths dictionary.
mincostpath = []
stateindex = np.argmin(costs[:,-1])
t = trellis.shape[1] - 1
while stateindex is not None:
mincostpath.insert(0, S[stateindex, :]) # Append state to front of path
stateindex = paths[(stateindex, t)]
t -= 1
# Convert into string format so we can use get_assembly() from question 2.3
# Also reverse the order so each string is "s(i-1)s(i)" instead of "s(i)s(i-1)"
mincostpathstringrev = [str(s[1]) + str(s[0]) for s in mincostpath]
message = np.array([int(i) for i in get_assembly(mincostpathstringrev)])
return message
y = np.array([1.34, 0.23, 1.45, 0.5, 0.11])
Q = np.array([0.9, 0.25])
S = np.array([[0, 0], [1, 0], [0, 1], [1, 1]])
print(ViterbiDecoding2mer(y, Q, S))
[1 1 0 1 0 0]
3.
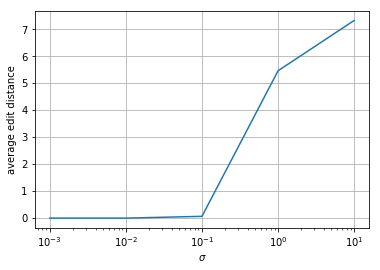
Simulate several length-20 \(s\). Use these to simulate \(y\) where \(n\) is sampled from a \(\mathcal{N}(0, \sigma^2)\) distribution. Decode \(y\) using the your Viterbi algorithm. Report the average edit distance between your recovered \(s\) and the actual \(s\) as a function of \(\sigma\). Test for \(\sigma =\) 1e-3, 1e-2, 1e-1, 1e0, 1e1.
import editdistance
L = 20
ntrials = 30
sigmas = [1e-3, 1e-2, 1e-1, 1e0, 1e1]
averagedists = np.zeros(len(sigmas))
for i in range(ntrials):
seq = np.random.randint(2, size=L)
for j, sigma in enumerate(sigmas):
# Generate random sequence
Qmat = np.zeros((L-1,L)) # Matrix to convert seqs to y
for i in range(L-1):
Qmat[i,i] = 0.25
Qmat[i,i+1] = 0.9
# Form noisy measurement matrix
y = np.dot(Qmat, seq) + sigma*np.random.randn(L-1)
recovered = ViterbiDecoding2mer(y, Q, S)
averagedists[j] += float(editdistance.eval(recovered, seq))
print(averagedists/ntrials)
plt.plot(sigmas, averagedists/ntrials)
plt.xlabel('$\sigma$')
plt.ylabel('average edit distance')
plt.xscale('log')
plt.grid()
plt.show()
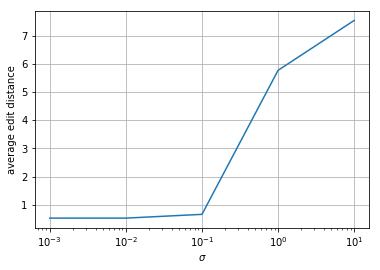
[0. 0. 0.06666667 5.46666667 7.33333333]
4.
Repeat 3 except randomly delete a base in \(s\) before generating your \(y\). What do you observe? From a high level, what might you need to change to fix this?
The edit distances for the small-error sequences all increase by a small amount. The high-error sequences are not as affected. From a high level, you might need to use longer kmers when doing Viterbi decoding so you can detect when things disappear like that (there will be more inter-symbol interference).
L = 20
ntrials = 30
sigmas = [1e-3, 1e-2, 1e-1, 1e0, 1e1]
averagedists = np.zeros(len(sigmas))
for i in range(ntrials):
seq = np.random.randint(2, size=L)
# Delete a random base
r = np.random.randint(len(seq))
seq2 = np.array(seq, copy=True)
seq2[r] = 0
for j, sigma in enumerate(sigmas):
# Generate random sequence
Qmat = np.zeros((L-1,L)) # Matrix to convert seqs to y
for i in range(L-1):
Qmat[i,i] = 0.25
Qmat[i,i+1] = 0.9
# Form noisy measurement matrix
y = np.dot(Qmat, seq2) + sigma*np.random.randn(L-1)
recovered = ViterbiDecoding2mer(y, Q, S)
averagedists[j] += float(editdistance.eval(recovered, seq))
print(averagedists/ntrials)
plt.plot(sigmas, averagedists/ntrials)
plt.xlabel('$\sigma$')
plt.ylabel('average edit distance')
plt.xscale('log')
plt.grid()
plt.show()
[0.53333333 0.53333333 0.66666667 5.76666667 7.53333333]